by Kristin Scaplen, Bryant University, [This article first appeared in The Conversation, republished with permission]
The human brain contains approximately 87 billion neurons. On average, each of these cells make thousands of different connections to facilitate communication across the brain. Neural communication is thought to underlie all brain functions – from experiencing and interpreting the world around you to remembering those experiences and controlling how your body responds.
But in this vast network of neural communication, precisely who is talking to whom, and what is the consequence of those individual conversations?
Understanding the details surrounding neural communication and how it’s shaped by experience is one of the many focuses of neuroscience. However, this is complicated by the sheer number of microscopic connections there are to study in the human brain, many of which are often in flux, and that available tools are unable to provide adequate resolution.
As a consequence, many scientists like me have turned to simpler organisms, such as the fruit fly.
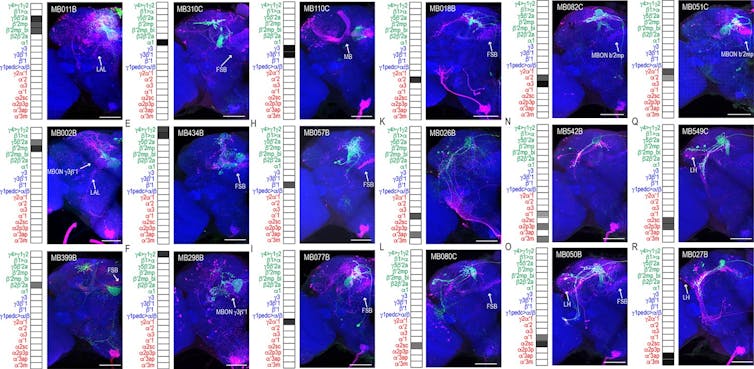
This figure shows connections between different mushroom body neurons. Scaplen et al. 2021/eLife, CC BY
Fruit flies, though pesky in the kitchen, are invaluable in the laboratory. Their brains are built in remarkably similar ways to those of humans. Importantly, scientists have developed tools that make fly brains significantly easier to study with a resolution that hasn’t been achieved in other organisms.
My colleague Gilad Barnea, a neuroscientist at Brown University, and his team spent over 20 years developing a tool to visualize all of the microscopic connections between neurons within the brain.
Neurons communicate with each other by sending and receiving molecules called neurotransmitters between receptor proteins on their surface. Barnea’s tool, trans-Tango, translates the activation of specific receptor proteins into gene expression that ultimately allow for visualization.
My team and I used trans-Tango to visualize all the neural connections of a learning and memory center, called the mushroom body, in the fruit fly brain.

The video starts close to the face of the fly and moves back, using genetics to express different proteins within neurons to visualize them. Green indicates the neuron of interest, red indicates the neuron it talks to and blue indicates all other brain cells. Kristin Scaplen, CC BY-SA
Here, a cluster of approximately four neurons, labeled green, receive messages from the mushroom body, which is the L-shaped structure labeled blue in the center of the fly brain. You can step through the brain and see all the other neurons they likely communicate with, labeled red. The cell bodies of the neurons reside on the edges of the brain, and the locations where they receive messages from the mushroom body appear as green tangles invading a small oval compartment. Where these weblike green extensions mingle with red are thought to be where neurons communicate their processed message to other downstream neurons.
Stepping further into the brain, you can see the downstream neurons navigating to a single layer of a fan-shaped structure within the brain. This fan-shaped body is thought to modulate many functions, including arousal, memory storage, locomotion and transforming sensory experiences into actions.
Not only did our images reveal previously unknown connections across the brain, but it also provides an opportunity to explore the consequences of those individual neural conversations. Fly brain connections were remarkably consistent but also varied slightly from one fly to another. These slight variations in connectivity are likely influenced by the fly’s individual experiences, just like they are in people.
The beauty of trans-Tango lies in its flexibility. In addition to visualizing connections, scientists can use genes to manipulate neural activity and better understand how neural communication affects behavior. Because fly brains are similarly built to those of humans, researchers can use them to study how brain connections function and how they might be disrupted in disease. Ultimately, this will improve our understanding of our own brains and the human condition.
Kristin Scaplen, Assistant Professor of Neuroscience, Bryant University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
